CELL MEMBRANE
The lipid bilayer
The lipid bilayer has been firmly established as the universal basis of
membrane structure, and its properties are responsible for the general
properties of all cell membranes. Because cells are filled with—and surrounded
by—solutions of molecules in water, we begin this section by
considering how the structure of cell membranes is a consequence of the
way membrane lipids behave in a watery (aqueous) environment.
Membrane lipids Form bilayers in Water
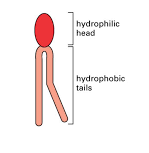
The lipids in cell membranes combine two very different properties in a
single molecule: each lipid has a hydrophilic (“water-loving”) head and
one or two hydrophobic (“water-fearing”) hydrocarbon tails.
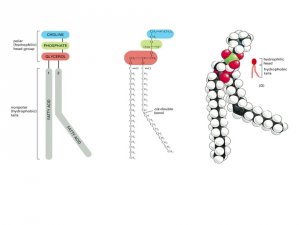
The most abundant lipids in cell membranes are the phospholipids,
molecules in which the hydrophilic head is linked to the rest of the lipid
through a phosphate group. The most common type of phospholipid
in most cell membranes is phosphatidylcholine, which has the small
molecule choline attached to a phosphate as its hydrophilic head and two
long hydrocarbon chains as its hydrophobic tails.
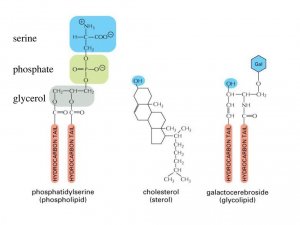
Molecules with both hydrophilic and hydrophobic properties are termed
amphipathic. This chemical property is also shared by other types of
membrane lipids, including the sterols (such as the cholesterol found in
animal cell membranes) and the glycolipids, which have sugars as part
of their hydrophilic head. Having both hydrophilic and
hydrophobic parts plays a crucial part in driving these lipid molecules to
assemble into bilayers in an aqueous environment.
Hydrophilic molecules dissolve readily in water
because they contain charged atoms or polar groups, that is, chemical
groups with an uneven distribution of positive and negative charges; these
charged atoms can form electrostatic attractions or hydrogen bonds with
water molecules, which are themselves polar. Hydrophobic
molecules, by contrast, are insoluble in water because all—or almost
all—of their atoms are uncharged and nonpolar; they, therefore, cannot
form favorable interactions with water molecules. Instead, these nonpolar
atoms force adjacent water molecules to reorganize into a cagelike
structure around the hydrophobic molecule. Because the
cagelike structure is more highly ordered than the surrounding water,
its formation requires energy. The energy cost is minimized, however, if
the hydrophobic molecules cluster together, limiting their contact with
water to the smallest possible number of water molecules. Thus, purely
hydrophobic molecules, like the fats found in animal fat cells and the oils
found in plant seeds, coalesce into a single large drop
when dispersed in water.
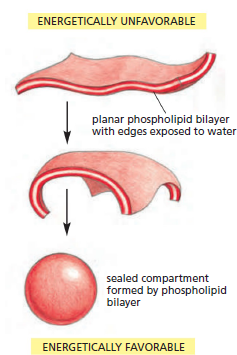
the molecules of the bilayer will spontaneously rearrange to eliminate
the free edge. If the tear is small, this spontaneous rearrangement will
exclude the water molecules and lead to repair of the bilayer, restoring
a single continuous sheet. If the tear is large, the sheet may begin to fold
in on itself and break up into separate closed vesicles. In either case, the
overriding principle is that free edges are quickly eliminated.
The prohibition on free edges has a profound consequence: the only way
a finite sheet can avoid having free edges is to bend and seal, forming a
boundary around a closed space. Therefore, amphipathic
molecules such as phospholipids necessarily assemble into self-sealing
containers that define closed compartments. This remarkable behavior,
fundamental to the creation of a living cell, is in essence simply a result
of the property that each molecule is hydrophilic at one end and hydrophobic
at the other.
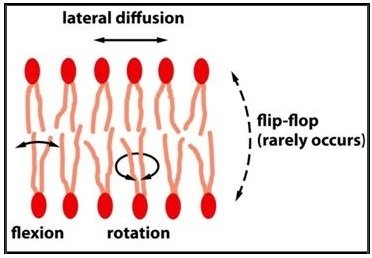
the lipid bilayer is a two-dimensional Fluid
The aqueous environment inside and outside a cell prevents membrane
lipids from escaping from the bilayer, but nothing stops these molecules
from moving about and changing places with one another within the
plane of the bilayer. The membrane therefore behaves as a twodimensional
fluid, which is crucial for membrane function and integrity
. this property is distinct from flexibility, which is the ability of
the membrane to bend. Membrane flexibility is also important, and it sets
a lower limit of about 25 nm to the size of vesicle that cell membranes
can form.
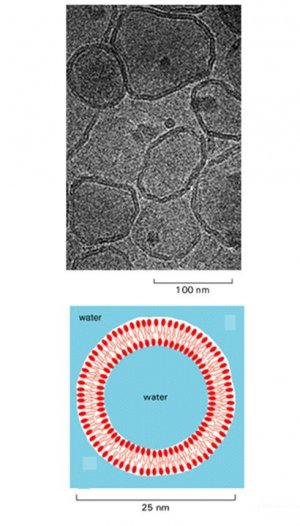
The fluidity of lipid bilayers can be studied using synthetic lipid bilayers,
which are easily produced by the spontaneous aggregation of amphipathic
lipid molecules in water. Two types of synthetic lipid bilayers are
commonly used in experiments. Closed spherical vesicles, called liposomes,
form if pure phospholipids are added to water; they vary in size
from about 25 nm to 1 mm in diameter. Alternatively, flat
phospholipid bilayers can be formed across a hole in a partition between
two aqueous compartments.
These simple artificial bilayers allow measurements of the movements
of the lipid molecules, revealing that some types of movement are rare
while others are frequent and rapid. Thus, in synthetic lipid bilayers,
phospholipid molecules very rarely tumble from one half of the bilayer,
or monolayer, to the other. Without proteins to facilitate the process
and under conditions similar to those in a cell, it is estimated that this
event, called ‘flip-flop,’ occurs less than once a month for any individual
lipid molecule. On the other hand, as the result of thermal motions, lipid
molecules within a monolayer continuously exchange places with their neighbors.
This exchange leads to rapid diffusion of lipid
molecules in the plane of the membrane so that, for example, a lipid in an artificial bilayer may diffuse a length equal to that of a large bacterial cell (~2 mm) in about one second. If the temperature is decreased, the drop in thermal energy decreases the rate of lipid movement, making the bilayer less fluid.
Similar results are obtained when one examines isolated cell membranes
and whole cells, indicating that the lipid bilayer of a cell membrane
also behaves as a two-dimensional fluid in which the constituent lipid
molecules are free to move within their own layer in any direction in the
plane of the membrane. These studies also show that lipid hydrocarbon
chains are flexible and that individual lipid molecules within a monolayer
rotate very rapidly about their long axis—some reaching speeds of 30,000
revolutions per minute . In cells, as in synthetic bilayers,
individual phospholipid molecules are normally confined to their own
monolayer and do not flip-flop spontaneously.
the Fluidity of a lipid bilayer depends on its Composition
The fluidity of a cell membrane—the ease with which its lipid molecules
move within the plane of the bilayer—is important for membrane function
and has to be maintained within certain limits. Just how fluid a lipid
bilayer is at a given temperature depends on its phospholipid composition
and, in particular, on the nature of the hydrocarbon tails: the closer
and more regular the packing of the tails, the more viscous and less fluid
the bilayer will be. Two major properties of hydrocarbon tails affect how
tightly they pack in the bilayer: their length and the number of double
bonds they contain.
A shorter chain length reduces the tendency of the hydrocarbon tails
to interact with one another and therefore increases the fluidity of the
bilayer. The hydrocarbon tails of membrane phospholipids vary in length
between 14 and 24 carbon atoms, with 18–20 atoms being most usual.
Most phospholipids contain one hydrocarbon tail that has one or more
double bonds between adjacent carbon atoms, and a second tail with
single bonds only. The chain that harbors a double bond
does not contain the maximum number of hydrogen atoms that could, in
principle, be attached to its carbon backbone; it is thus said to be unsaturated
with respect to hydrogen. The fatty acid tail with no double bonds has a full complement of hydrogen atoms; it is said to be saturated. Each double bond in an unsaturated tail creates a small kink in the hydro-carbon tail, which makes it more difficult for the tails
to pack against one another. For this reason, lipid bilayers that contain
a large proportion of unsaturated hydrocarbon tails are more fluid than
those with lower proportions.

In bacterial and yeast cells, which have to adapt to varying temperatures,
both the lengths and the unsaturation of the hydrocarbon tails in the
bilayer are constantly adjusted to maintain the membrane at a relatively
constant fluidity: at higher temperatures, for example, the cell makes
membrane lipids with tails that are longer and that contain fewer double
bonds. A similar trick is used in the manufacture of margarine from
vegetable oils. The fats produced by plants are generally unsaturated and
therefore liquid at room temperatures, unlike animal fats such as butter
or lard, which are generally saturated and therefore solid at room temperature.
Margarine is made of hydrogenated vegetable oils; their double
bonds have been removed by the addition of hydrogen so that they are
more solid and butterlike at room temperature.
In animal cells, membrane fluidity is modulated by the inclusion of the
sterol cholesterol. This molecule is present in especially
large amounts in the plasma membrane, where it constitutes approximately
20% of the lipids in the membrane by weight. Because cholesterol
molecules are short and rigid, they fill the spaces between neighboring
phospholipid molecules left by the kinks in their unsaturated hydrocarbon
tails. In this way, cholesterol tends to stiffen the
bilayer, making it more rigid and less permeable. The chemical properties
of membrane lipids—and how they affect membrane fluidity.
For all cells, membrane fluidity is important for many reasons. It enables
membrane proteins to diffuse rapidly in the plane of the bilayer and to
interact with one another, as is crucial, for example, in cell signaling. It permits membrane lipids and proteins to diffuse
from sites where they are inserted into the bilayer after their synthesis to
other regions of the cell. It allows membranes to fuse with one another
and mix their molecules, and it ensures that membrane molecules are
distributed evenly between daughter cells when a cell divides. If biological
membranes were not fluid, it is hard to imagine how cells could live,
grow, and reproduce.
Cell membranes are generally asymmetrical: they present a very different
face to the interior of the cell or organelle than they show to the exterior.
The two halves of the bilayer often include strikingly different sets ofphospholipids and glycolipids. Moreover, membrane proteins
are embedded in the bilayer with a specific orientation, which is
crucial for their function.
the lipid bilayer is asymmetrical
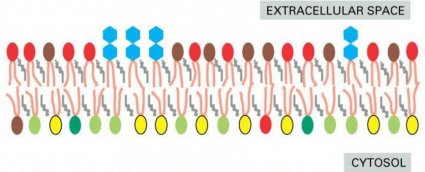
lipid asymmetry is preserved during Membrane transport
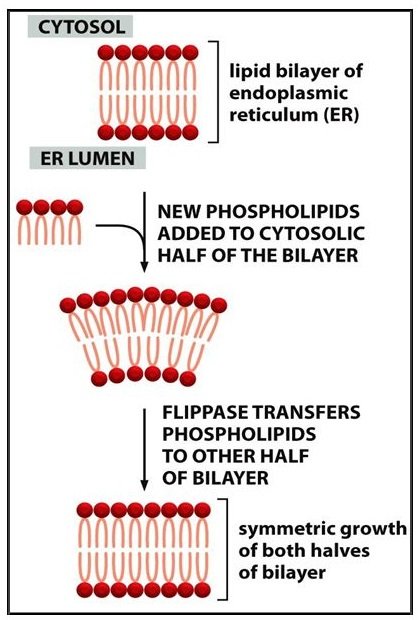
The lipid asymmetry is established and maintained as the membrane
grows. In eucaryotic cells, new phospholipids are manufactured by
enzymes bound to the part of the endoplasmic reticulum membrane that
faces the cytosol. These enzymes, which use free fatty acids as substrates
, deposit all newly made phospholipids into the
cytosolic half of the bilayer. To enable the membrane as a whole to grow evenly, half of the new phospholipid molecules then have to be transferred to the opposite monolayer. This transfer is catalyzed by enzymes called flippases . In the plasma membrane, flippases transfer specific phospholipids selectively, so that different types become concentrated in each monolayer.
Using selective flippases is not the only way to produce asymmetry in
lipid bilayers, however. In particular, a different mechanism operates for
glycolipids—the lipids that show the most striking and consistent asymmetric
distribution in animal cells. To explain their
distribution, it is necessary to take a more detailed look at how new
membrane is produced in eucaryotic cells.
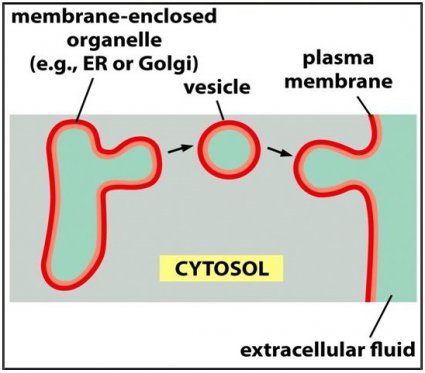
Nearly all new membrane synthesis in eucaryotic cells occurs in the
membrane of one intracellular compartment—the endoplasmic reticulum
.
The new membrane assembled there is exported to the other membranes
of the cell through a cycle of membrane budding and fusion: bits of the
bilayer pinch off from the ER to form small spheres called vesicles,
which then become incorporated into another membrane,
such as the plasma membrane, by fusing with it. The orientation
of the bilayer relative to the cytosol is preserved during vesicle
formation and fusion. This preservation of orientation means that all cell
membranes, whether the external plasma membrane or an intracellular
membrane around an organelle, have distinct ‘inside’ and ‘outside’ faces
that are established at the time of membrane synthesis: the cytosolic face
is always adjacent to the cytosol, while the noncytosolic face is exposed
to either the cell exterior or the interior space of an organelle.
Glycolipids are located mainly in the plasma membrane, and they are
found only in the noncytosolic half of the bilayer. Their sugar groups are
therefore exposed to the exterior of the cell, where they
form part of a continuous protective coat of carbohydrate that surrounds
most animal cells. The glycolipid molecules acquire their sugar groups
in the Golgi apparatus, the organelle to which proteins and membranes
made in the ER often go next . The enzymes that
add the sugar groups are confined to the inside of the Golgi apparatus, so
that the sugars are added only to lipid molecules in the noncytosolic
half of the lipid bilayer. Once a glycolipid molecule has been created in this
way, it remains trapped in this monolayer, as there are no flippases that
transfer glycolipids to the cytosolic monolayer. Thus, when a glycolipid
molecule is finally delivered to the plasma membrane, it faces away from
the cytosol and displays its sugar on the exterior of the cell.
Other lipid molecules show different types of asymmetric distributions,
related to other functions. The inositol phospholipids, for example, are
minor components of the plasma membrane, but they play a special role
in relaying signals from the cell surface to the intracellular components
that respond to those signals. They act only
after the signal has been
transmitted across the plasma membrane; thus they are concentrated
in the cytosolic half of this lipid bilayer.
and others to be exported. Other proteins in the membrane act as sensors that enable the cell to receive information about changes in its environ- ment and respond to them (Figure 11–2). The mechanical properties of the
membrane are equally remarkable. When a cell grows or changes shape,
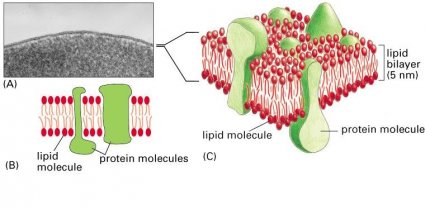
The simplest bacteria have only a single membrane—the plasma mem- brane. Eucaryotic cells, however, also contain an abundance of internal membranes that enclose intracellular compartments to form the various organelles, including the endoplasmic reticulum, Golgi apparatus, and mitochondria (Figure 11–3). These internal membranes are constructed on the same principles as the plasma membrane, and they, too, serve as highly selective barriers between spaces containing distinct collections of molecules (see Figure 11–1B). Subtle differences in the composition of these membranes, especially in their resident proteins, give each organelle its distinctive character.
Regardless of their location, all cell membranes are composed of lipids and proteins and share a common general structure (Figure 11–4). The lipids are arranged in two closely apposed sheets, forming a lipid bilayer (see Figure 11–4B and C). This lipid bilayer gives the membrane its basic structure and serves as a permeability barrier to most water-soluble mol- ecules. The proteins carry out most of the other functions of the membrane and give different membranes their individual characteristics.
In this chapter we consider the structure of biological membranes and the organization of their two main constituents: lipids and proteins. Although we focus mainly on the plasma membrane, most of the concepts we dis- cuss also apply to internal membranes. The functions of cell membranes, including their role in cell communication, the transport of small mol- ecules, and energy generation, are considered in later chapters.
Back Next
 Magazine Posts
Magazine Posts Table of Contents
Table of Contents